07-03-2020
Cristiano Mota and Maria João Romão, from the Macromolecular Crystallography Lab at UCIBIO-FCT NOVA, in collaboration with Ana Rita Oliveira and Inês Cardoso Pereira from ITQB NOVA, have identified a protein capable of reducing carbon dioxide (CO2) from the atmosphere. The results published in the scientific journal ACS Catalysis indicate new ways to solve one of the biggest current challenges for the planet's sustainability: carbon neutrality.
While the levels of CO2 in the Earth's atmosphere reach record levels in the history of mankind, the European Union is committed to achieving neutrality in carbon emissions by 2050. In order to reduce global emissions of greenhouse gases, these emissions have to be balanced with the capture of CO2. Plants, soils and oceans are some of the responsible for this removal, but not sufficient to achieve the objective. It is therefore urgent to find new catalysts that allow the transformation (reduction) of CO2 into other added-value products.
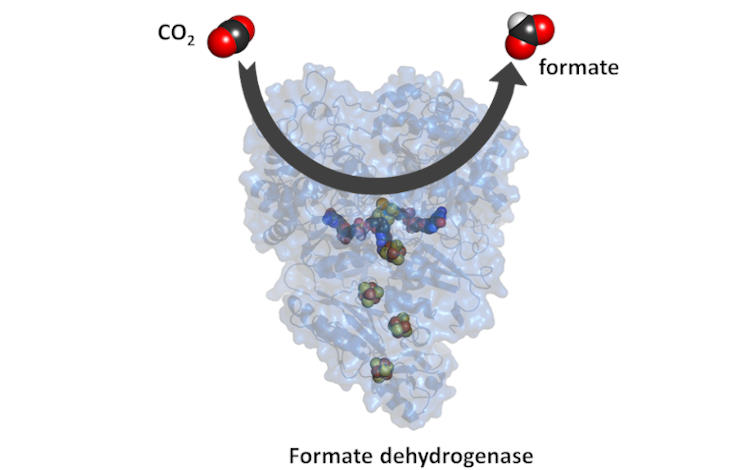
In the search for solutions to reduce atmospheric CO2, the catalytic efficiency of microorganisms can be explored. One option is to use bacteria whose natural metabolism involves CO2 reduction. This process involves a type of enzymes, called formate dehydrogenases (Fdhs), which transform CO2 into formate, a chemical fuel equivalent to hydrogen. These enzymes are found in one of the oldest and most energy efficient biological pathways. "Understanding the mechanism of this biological process with billions of years of evolution can give us the key to the development of new technologies that allow the reduction of atmospheric levels of carbon dioxide", explains Inês Cardoso Pereira, who led the study at ITQB NOVA.
In the work now published in the prestigious journal ACS Catalysis, the researchers focused on understanding the CO2 reduction mechanism. For this, they studied the formate dehydrogenase from a bacterium that is quite common in soils and marine environments, but exists also in the human intestine: Desulfovibrio vulgaris. Through an innovative study, it was possible to understand that this protein is very active in reducing CO2 and very stable in the presence of oxygen, a useful and unusual feature. In addition, the determination of its three-dimensional structure in different stages of the catalysis was a decisive step towards understanding the CO2 reduction mechanism. “These characteristics make this enzyme an ideal model for the study of this mechanism and for the development of similar synthetic catalysts”, adds Maria João Romão, leader of this study at UCIBIO-FCT NOVA and also Director of the research unit UCIBIO. “The molecular understanding of this mechanism and the characterization of these enzymes provide important clues for the biotechnological advance in the development of strategies and systems for the fixation of carbon dioxide”, explains Cristiano Mota, one of the first authors of the study.
The research teams led by Maria João Romão at UCIBIO-FCT NOVA and Inês Cardoso Pereira at ITQB NOVA, will now modify the enzyme to improve or reinforce its characteristics, making it even more interesting for use in the market. These developments reinforce the feasibility of using bio-hybrid systems in the sustainable decarbonization strategy.
Artigo:
ACS Catalysis, DOI: 10.1021/ACSCATAL.0C00086
Towards the mechanistic understanding of enzymatic CO2 reduction
Ana Rita Oliveira, Cristiano Mota, Cláudia Mourato, Renato M. Domingos, Marino F. A. Santos, Diana Gesto, Bruno Guigliarelli, Teresa Santos-Silva, Maria João Romão e Inês Antunes Cardoso Pereira
Nas notícias: