12-12-2017
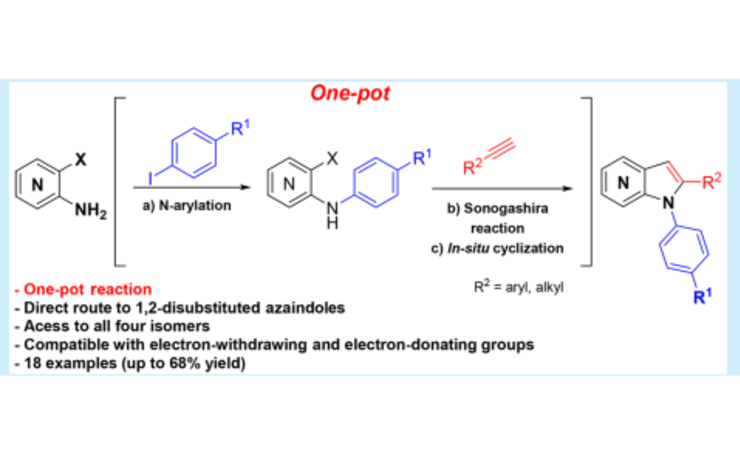
Azaindoles are bioisosteres of the indole scaffold, a privileged structure, and have been used in diverse areas of research, such as materials and medicinal chemistry due to their physiochemical and pharmacological properties. Substituted azaindoles are interesting drug discovery scaffolds because their properties can be mediated by changing the substitution pattern or the position of the endocyclic nitrogen. Efforts toward efficient strategies for the synthesis of substituted azaindoles have been developed, such as 2,3-and 1,4-disubstituted azaindoles. Despite this, 1,2-disubstituted azaindoles are almost unexplored, especially 1,2-diaryl azaindoles.
Maria Manuel Marques, principal investigator at LAQV, and co-workers at the Faculdade de Ciências e Tecnologia - Universidade Nova de Lisboa, detailed an efficient protocol to attain 4-, 5-, 6-, and 7-azaindoles in one pot (Org. Lett. 2017 , 19, 5118; DOI: 10.1021/acs.orglett.7b02403).
The described protocol consists of sequential N-arylation of amino-o-halopyridines, Sonogashira reaction, and cyclization to the desired product. This is a fast and elegant access to this challenging class of compounds and creates a new platform to access novel drug candidates or scaled synthesis of actual drugs.
This work has been highlighted by the journal Synfacts (Thieme Journals, Synfacts 01122017, 13(12), 1251; DOI: 10.1055/s-0036-1591687) and by the journal Organic Process and Research Development (ACS Journal; December issue; DOI: 10.1021/acs.oprd.7b00371).